Our Pipeline
Pioneering Hope in Rare Diseases
Our innovative research consists of a growing portfolio of highly promising programs offering treatments and life-changing opportunities for patients worldwide.
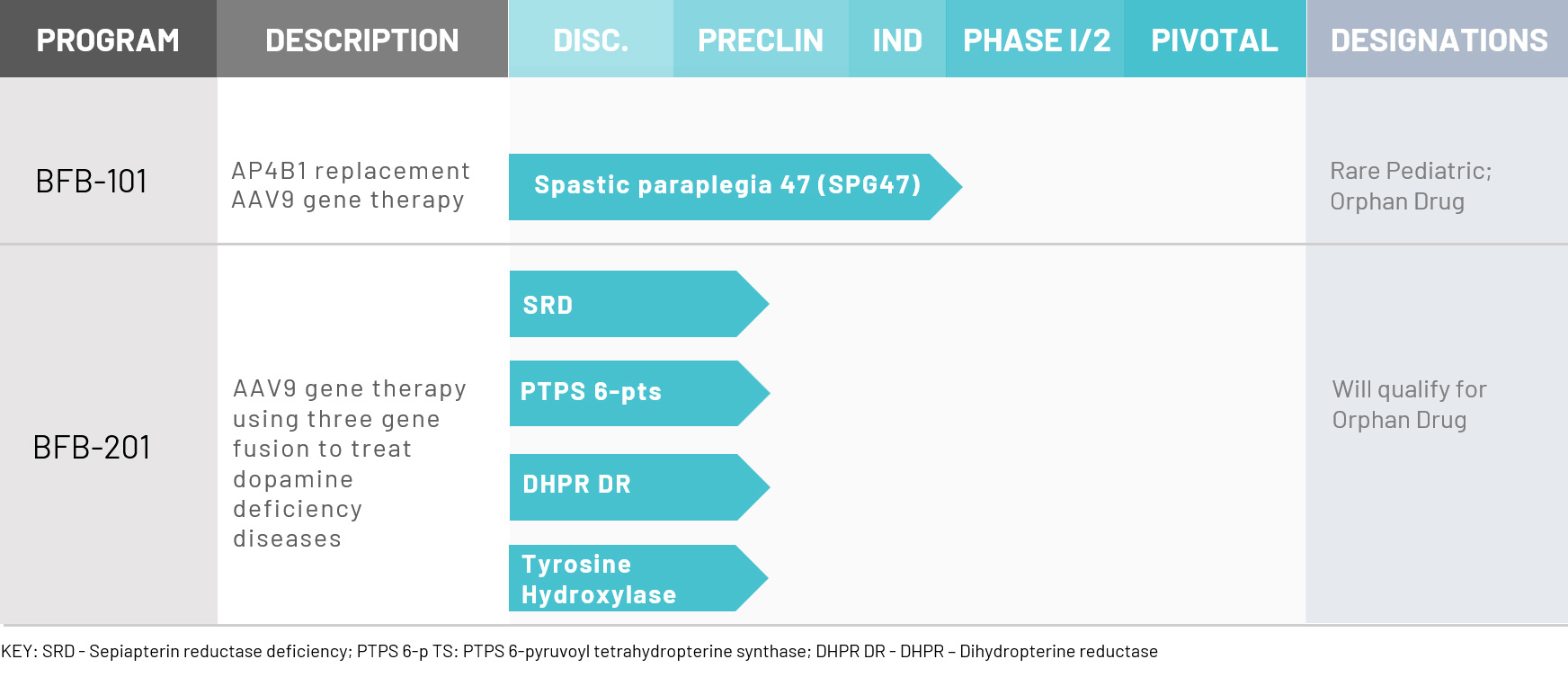
BFB-101: Spastic Paraplegia 47 (SPG47)
SPG47 is a rare form of AP-4 Hereditary Spastic Paraplegia (AP-4 HSP) with an estimated prevalence in excess of 5,000 patients worldwide. It’s a progressive neurodevelopmental and neurodegenerative disease characterised by early childhood spasticity, microencephaly and seizures.
The science behind the disease
The AP4B1 gene helps to move things inside our cells, specifically from the trans-Golgi network to the endosomal compartment. When this gene does not work correctly, we call it a loss-of-function mutation, which causes SPG47.
A potential solution
BFB-101 is a patented AAV vector that expresses the AP4B1 gene, developed by Professor Mimoun Azzouz at the University of Sheffield with the support of Cure AP-4 and LifeArc.
It is administered via the brain as a single lifetime dose and has shown potential in restoring AP-4 function in vitro and improving motor function in AP4B1 mutant mice.
Next stages
US FDA clearance to commence an inaugural clinical trial in SPG47 patients was received in Q2 2025. It is currently envisaged that recruitment for the Phase I/II trial will commence at Boston Children Hospital before end 2025. For more information on the trial, visit ClinicalTrials.gov.
BFB-201: Dopamine Replacement as a Therapeutic Strategy
A depletion in dopamine has been shown to be a cause of many serious neurological diseases including Parkinson’s disease, restless leg syndrome, ADHD and depression, with a number of treatments available for several of these diseases.
However, following a detailed investigation, the company has identified the following rare neurological diseases caused by dopamine deficiency for which no treatment exists: sepiapterin reductase deficiency (SRD), 6-pyruvoyl tetrahydropterine synthase deficiency (PTPSD), dihydropterine reductase (DHPR) and tyrosine hydroxylase (TH). The company intends to focus initially on these four diseases with its novel gene therapy program BFB-201.
A potential solution
BFB-201 is an adeno-associated virus (AAV) based gene therapy in preclinical development to treat these rare diseases in a one-time treatment administered to the brain. As these rare diseases are clinically similar, they could likely be treated as a single disorder group during clinical development.
BFB-201 is designed to uniquely deliver a single gene fusion cassette required for optimal dopamine synthesis. Administering these specific genes together in an adeno-associate virus would represent a scientific and clinical breakthrough, offering improved outcomes for patients and addressing the limitations of other dopamine replacement strategies e.g. lack of efficacy and off-target effects of L-Dopa treatment.
Clinical validation already exists as others have previously shown in clinical studies in other dopamine deficiency settings delivering these genes together is safe and efficacious in 21 patients for over 10 years.
Next stages
The company will complete in vitro and in vivo proof of concept with its proprietary gene fusion cassette before conducting the required toxicology studies to support initial clinical trials.
